Background: Ageing is a risk factor for cancer. Worldwide, the number and proportion of adults aged ≥65 will increase, along with the incidence of ovarian cancer. Older adults are under-represented in randomised clinical trials (RCTs), and those who are enrolled have a good performance status and no major health issues. These patients are not representative of older patients seen in everyday clinical practice; therefore, age-specific data on efficacy and toxicity of olaparib in the ‘real-world’ setting are lacking.
Methods: This observational study was conducted in the Central Jutland Region in Denmark. Data in unselected older (≥65) patients with known BRCA mutation receiving olaparib maintenance treatment for platinum-sensitive relapsed ovarian cancer were registered between 2015 and 2019. Toxicity and progression-free survival (PFS) were registered. No geriatric assessment has been performed.
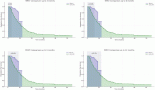
Results: In total, 20 consecutive patients ≥65 years were included with a median age of 75 years (range: 65–85). Most of the patients (18/20) had ECOG PS: 0–1. Treatment interruption and dose reduction occurred in 65% of the patients. Toxicities of any grade occurred in 18 (90%), whereas grade 3/4 toxicities occurred in 6 patients (30%). Treatment was terminated due to disease progression or unacceptable toxicity in 13 (65%) patients. The median PFS was 6 months (range: 2–31), and the median follow-up was 15 months (range: 3–30).
Discussion: Our ‘real-world’ experience shows that unselected older patients represent a significant larger proportion in real life than in RCTs; furthermore, older patients in a real-world setting may experience more side effects possibly affecting the quality of life. The median PFS data suggest that older patients may not derive the same clinical benefit than their fit and younger counterparts.
There is a need to enrol vulnerable/frail older patients into RCTs, ensuring that data will also be applicable in standard clinical settings. Incorporating geriatric assessment into these trials should be encouraged.